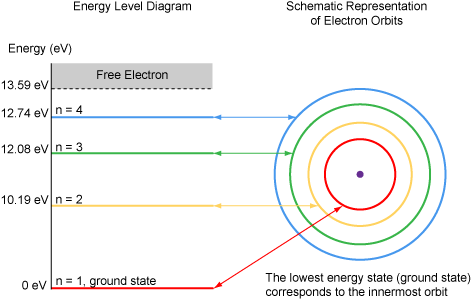
One important outcome of the Bohr model was the recognition of electron energy levels. Electrons can only orbit the nucleus at very specific energy levels. When electrons are in orbit close to the nucleus, they have lower energy, and when they orbit further from the nucleus, they have increased energy.
The energy states of the electrons in an atom are best shown using an energy level diagram. Hydrogen has the simplest structure of all of the elements (one proton and one electron). For this reason, the energy level diagram for Hydrogen will be presented first. The energy associated with each possible orbit is shown in the energy level diagram.
Ground State (n=1)
- In a neutral, unexcited atom, the electron is in the lowest energy state or ground state
- The ground state corresponds to the orbit closest to the nucleus.
Excited States (n = 2, 3, 4, ...)
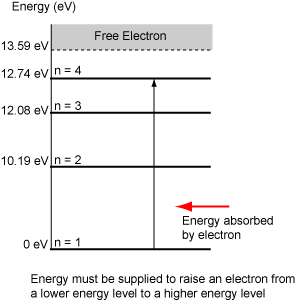
- The excited states in hydrogen are labelled n = 2, 3, 4, ...
- Energy must be supplied from an external source (heat, electric field, charged particle, photon, etc.) to lift the electron from the ground state to an excited state.
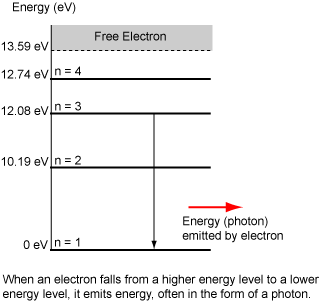
- When an electron is in an excited state, it will tend to fall back down to a lower energy state. When it does fall, it releases energy, often in the form of a photon.
Ionization Energy
- The energy to completely remove an electron from an unexcited atom is called the ionization energy. The ionization energy for hydrogen is 13.59 eV.
Binding Energy
The binding energy of an electron is the energy required to remove an electron from its specific shell of the atom. The binding energy of an electron in the ground state is equal to the ionization energy. The binding energy of excited electrons is less than the ionization energy of the atom.